Introduction to Quantum Mechanics: Particle or Wave – Both!

 For most of us, it is natural to think of atoms and electrons as particles. Light, on the other hand, is often described as a wave and is therefore seemingly quite different from an electron. But can something be a wave and a particle at the same time?
For most of us, it is natural to think of atoms and electrons as particles. Light, on the other hand, is often described as a wave and is therefore seemingly quite different from an electron. But can something be a wave and a particle at the same time?
The experimental test
Physics is, ultimately, an experimental science in the sense that what is true about the world around us is determined by observation and measurements. However, that does not mean that theory is obsolete. Far from it, theoretical physics is indispensable in the task of understanding the behavior of nature because it both
- Predicts new phenomena which may subsequently be experimentally verified.
- Explains new experimental measurements which are not yet understood.
However, a theory can only be regarded as (potentially) correct as long as it is consistent with experimental measurements. It was for this reason that the demise of classical physics on microscopic length scales started to become apparent at the end of the 19th century. A set of experimental measurements were reported which were inconsistent with the predictions of classical physics.
Hence, a need was established for a new theory that would be consistent with the experimental observations. This was the seed that at the beginning of the 20th century eventually would grow into the theory of quantum physics.
Challenging our intuition
One of the most fascinating and fundamental concepts in quantum physics is that light can act both as a particle and a wave, and so can atoms and electrons! The classical and familiar concepts of “particles” and “waves” are on their own simply unable to describe fully the world on a microscopic level. This truly challenges our understanding of what light and matter are, but fortunately, quantum mechanics presents a beautiful theory which explains how this can be.
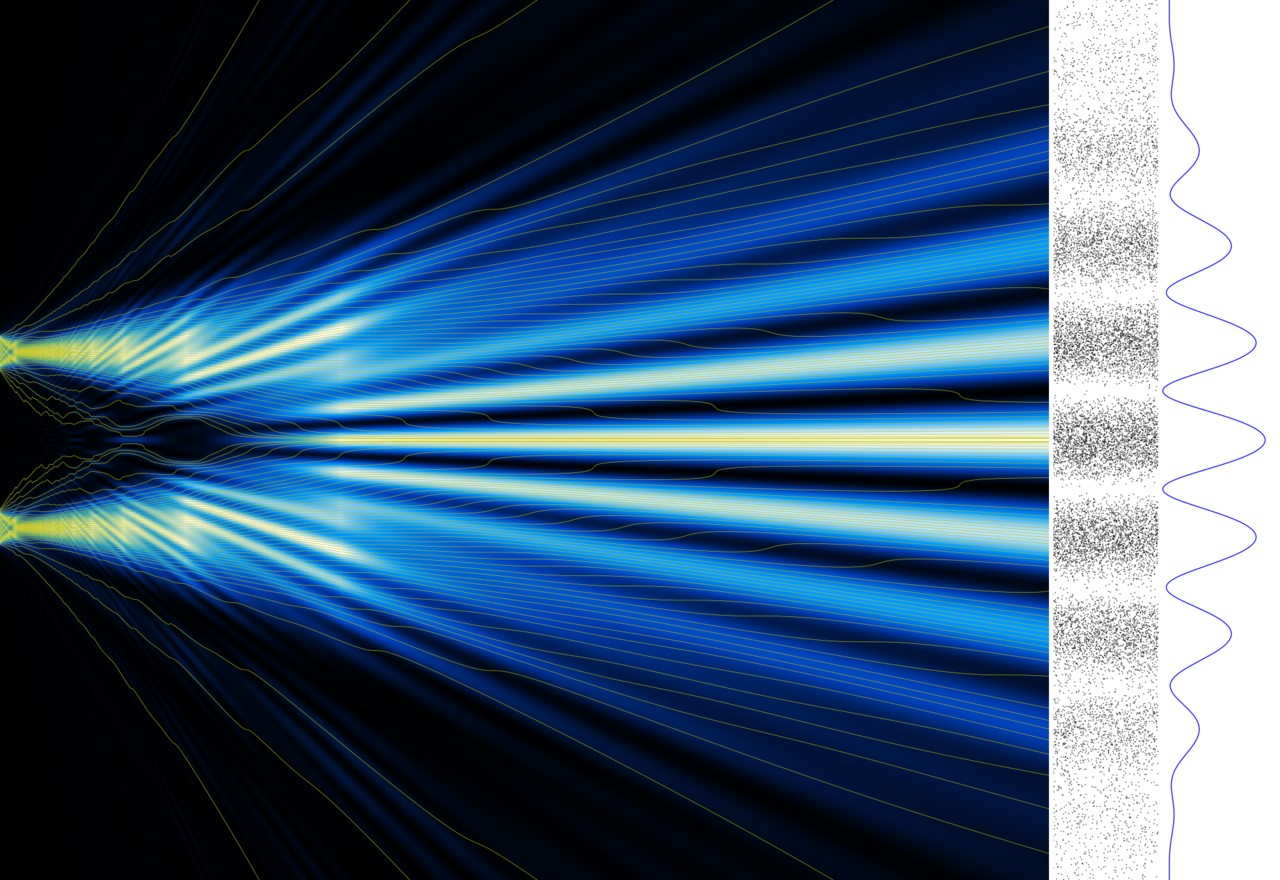
Experiments clearly show how electrons can act as waves. One way to illustrate this is to shoot electrons toward a wall with two small slits. If the electrons were simply particles, they would pass through either of the two slits and then hit a detector screen on the other side. One would simply see two points on the screen where the electrons hit it. But instead, the distribution of electrons on the screen is experimentally seen to display an interference pattern – just like if the electron acted like a wave (see figure below).
Reference: Image by Alexandre Gondran (from Wikimedia Commons)
Learn more
The eBook Introduction to Quantum Mechanics provides the reader with an introduction to the fundamental concepts of quantum physics and explains the particle-wave duality that exists in Nature. The reader is guided through how one can make sense out of the strange and seemingly counterintuitive, such as the interference pattern of electrons shown above, which are well documented experimentally.
This book will also serve as a fundament which allows the reader to later move on to more advanced topics in theoretical physics. The reader can expect to be capable of performing basic quantum mechanical calculations after studying this eBook, describing phenomena such as quantum mechanical tunneling, exchange forces, and the application of quantum mechanics to solid materials.